Earlier today the
BBC reported on the concerns surrounding an anti-malaria drug.
Lariam (mefloquine) is said to be causing concern regarding its side-effects, of which suicidal thinking and completed suicide are listed.
Worryingly, Lariam is handed out to soldiers who train or are in combat in countries where malaria is rife.
Surely, a pill taken to prevent malaria can't be that bad?
Think again.
I accessed the MHRA's Drug Analysis Prints (DAP's) earlier today - the results for Lariam were startling. Question is, why have the British drug regulator overlooked the glaringly obvious?
In all, there have been 19 reported fatalities regarding Lariam. Okay, not a great deal but when you look into the DAP's you will find that 9 of these 19 deaths have been death by suicide.
One thing the MHRA have always played down with antidepressant related suicide is that it could be the "illness" that caused the suicide and not the product. Fair enough, I guess but in the case of Lariam, the patients subjected to this particular drug do so because they wish to prevent something and not suppress or cure something.
Let's dig a little deeper.
Lariam was created by the military in the 1970's and manufactured by pharmaceutical company, Hoffman-La Roche.
Lariam Tablets contain the active ingredient mefloquine. They are used to treat malaria and to help prevent you from catching malaria.
Drug Analysis Print for Lariam (MHRA)
- Total number of reactions 6713
- Total number of ADR reports: 2282
- Total number of fatal ADR reports: 19
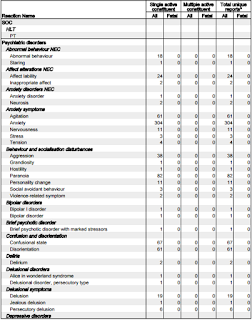
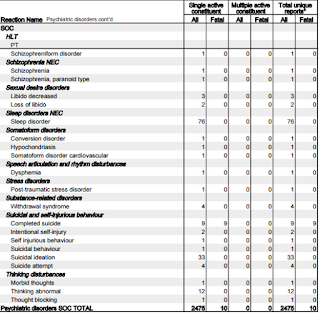
Let's focus on the adverse reactions associated with 'psychiatric disorders.'
There here have been...
- 18 reports of Abnormal behaviour.
- 24 reports of Affect lability (rapid shifts in outward emotional expressions; often associated with organic brain syndromes such as intoxication.)
- 61 reports of Agitation
- 304 reports of Anxiety
- 38 reports of Aggression
- 82 reports of Paranoia
- 11 reports of Personality change
- 67 reports of Confusional state
- 61 reports of Disorientation
- 19 reports of Delusion
- 341 reports of Depression
- 10 reports of Depersonalisation
- 14 reports of Dissociation
- 28 reports of Irritability
- 59 reports of Mood swings
- 15 reports of Mental disorder
- 11 reports of Mania
- 162 reports of Panic attack
- 119 reports of Abnormal dreams
- 146 reports of Nightmares
- 113 reports of Hallucinations
- 19 reports of Acute psychosis*
- 76 reports of Psychotic disorder
- 9 reports of Completed suicide
- 33 reports of Suicidal ideation
- 12 reports of Thinking abnormal
* Bizarrely, there has been one reported death of "acute psychosis" - This seems to have been omitted from the "completed suicide" catagory - unless, of course, the person died naturally?
So, a whole heap of psychiatric disorders are being reported to the British drug regulator. They, as usual, log these reports and make them public to geeks like me.
What is worrying here is that Lariam is the standard drug used for British soldiers training or fighting wars in countries that has a presence of malaria.
So, just to spell it out for the limpless drug regulator.
A drug causing or apparently causing all of the above is given to men and women who, as part of their training and/or duty of combat, are given guns and live ammunition.
All of the above reports were spontaneous reports sent through the Yellow Card System, a system that the MHRA are proud of. How many, if any at all, have the MHRA investigated?
A question I put to them under the Freedom of Information Act.
Dear Sirs,
According to the MHRA's DAP for the drug MEFLOQUINE there have been 19 reported fatalities associated.
Whilst I understand that this does not mean the drug caused the fatalities I would, however like to draw your attention to the number of people that have died by suicide whilst taking MEFLOQUINE
Your own figures suggest that, out of the 19 reported fatalities, 9 have been by completed suicide. This is significantly high for a drug used to treat malaria.
Under the terms of the freedom of information act, I would like to know how many of these 9 reports the MHRA have investigated and what were the outcomes of these investigations.
Furthermore, there have been 33 reports of suicide ideation, and 4 suicide attempts. The Psychiatric disorders reported for this particular drug are quite staggering. Again, Under the terms of the freedom of information act, I would like to know how many reports of Psychiatric disorders have been followed up by the MHRA.
Sincerely,
Bob Fiddaman
--
I am quite flabbergasted at these figures.
This, ladies and gentlemen, is the MHRA at work. They collect adverse reports (Yellow Cards) and file them. Aren't they supposed to protect the public from unsafe drugs?
How is a system that reports adverse reactions protecting the public? What are the MHRA doing regarding investigating the 9 suicides (possibly 10)?
It seems absurd that a drug given to healthy patients (soldiers) is still on the market when reports of it are possibly the cause of all of the above psychiatric disorders.
The MHRA have 20 working days to reply to my FOI request.
I will, of course, publish any reply they give to me. It's a matter of public interest.
DAP'S
Bob Fiddaman.