Wendy Dolin
Wendy Dolin's name has been cemented in history, as has that of her late husband, Stewart Dolin.
Her
victory against pharmaceutical giant, GlaxoSmithKline, was never simply about Paxil causing the death of an adult. Many people in my circles already knew Paxil can and does cause akathisia and death among people of all ages. But GlaxoSmithKline and its paid experts have played down this truth for many years. Other pharmaceutical companies that manufacture SSRIs, (Pfizer-Zoloft, Eli Lilly-Prozac, etc.) have also actively concealed that akathisia is a serious adverse side effect of their products.
Wendy's husband needlessly died as a result of GlaxoSmithKline failing to warn about akathisia. Furthermore, GSK failed to warn that akathisia can lead to suicide.
I started writing about Wendy's case two years ago. I never knew back then the impact it would have on me as a writer, a consumer, and a human. It was inevitable that our paths would cross. Most of the stories on my blog feature real people harmed by drugs the pharmaceutical companies call "antidepressants." These people are not fictional characters; their loved ones are not simply statistics. All are real people who, as a result of corporate greed and deceit, lost a family member. I have personally met the majority of the families featured on my blogs, families who, through no fault of their own, have been left devastated by unimaginable, avoidable deaths. These courageous family members are left to pick up the pieces of a life obliterated by the pharmaceutical industry and its incestuous relationships with medicine regulators, such as the FDA and MHRA.
My own personal belief is that pharmaceutical CEO's and executives should be imprisoned for withholding important safety information from consumers, particularly when withholding such critical information leads to suffering and/or death. This was surely the case in Dolin Vs GlaxoSmithKline. I also believe experts called to defend products in pharmaceutical litigation should be imprisoned if the evidence they produce at trial is shown to be false and if it is deemed perjury. One only has to look through the court transcripts in this case to see several statements by experts that were simply untrue.
Wendy, her family and close circle of friends, have remained dignified throughout the trial and pre-trial. On the other hand, the tactics of GlaxoSmithKline's hired attorneys, King & Spalding, has been nothing short of repugnant. If their pre-trial tactics were legal, then the legal system needs a complete overhaul. Wendy's interview today shares some of the shenanigans GSK pulled years ago before the trial began.
I have a particular disdain for King & Spalding, probably more so than GlaxoSmithKline. That disdain has been strengthened after interviewing Wendy and, of course, after being present during the first two weeks of this trial.
I think it's safe to assume King & Spalding don't like me either. I can live with that safe in the knowledge that I am, in essence, trying to do part of a job coroners should be doing. I try to give the dead voice. King & Spalding, it appears, not only try to stifle the voices of the living, but they also try to suppress and manipulate the voices of the dead.
I'm really proud of Wendy and her children, just as I am of others who take on the mighty pharmaceutical industry, be it through lawsuits, blogging or other advocacy work. It's a dark, seedy world. I should know, I've been writing and researching about Big Pharma for more than ten years.
~ Bob Fiddaman
Here's my interview with Wendy Dolin.
Congratulations on last week's successful trial against GSK. You worked tenaciously since filing the case in 2014. I imagine today's feelings of victory are bittersweet for you and your family.
Many people are unaware how traumatic the pre-trial process can be when challenging pharmaceutical companies and their attorneys. Can you share your experiences?
I knew when I filed this lawsuit, it was going to be a very difficult process. But I was unprepared for the sheer number of depositions and subpoenas GSK demanded. I was told this was a record number of requests. I understood the need for certain information, but it became very clear early on that GSK's goal was to send a powerful message to me: That is, when you have the audacity to challenge GSK, all attempts will be made to harass everyone you care dearly about. GSK also repeatedly tried to humiliate me. For example, depositions that should have been a few hours became eight hours in an attempt to wear people down. GSK asked the same question over and over and over again hoping to manipulate, confuse and take people's comments out of context.
Some of the irrelevant but personal questions GSK asked me included,
"How many times do you go to temple? Are you dating anyone? Who are my partners at work?" They even requested Stewart's high school transcripts. All were totally irrelevant and useless questions posed by attorneys from King and Spalding and Dentons. They were calling my friends, not identifying themselves and trying to get people to somehow say terrible things about my relationship with Stewart. There was nothing to say, of course, and GSK's attorneys just embarrassed themselves. It became a joke amongst my friends as to who would be called next and who did GSK think they were dealing with that they thought their sweet talking female attorney was somehow going to get information?
All of these questions were offensive, but what is truly the most offensive and egregious act was showing my children Stewart's therapy notes during depositions. As a therapist, as a mother and a compassionate human being, I am aware there was no purpose to have done such. I have talked to therapists, physicians and pharmaceutical lawyers and all agree there was nothing gained by this other than to show me that GSK would stop at nothing to intimidate me.
So, let me get this straight, attorneys for GSK telephoned your friends to try and dig up dirt on you? What sort of questions were they asking your circle of friends?
The good news regarding the phone calls is that most of my friends very shortly into the conversations realized something wasn't quite right, and therefore they shortly ended the conversations. They asked
"Do you know Wendy and Stewart Dolin?" or
"What can you tell us about Wendy and Stewart Dolin's relationship?" Most people said,
"Whose side are you on?" To which, GSK attorneys replied,
"You could be getting a subpoena, and that is not a very pleasant experience, so maybe you would like to tell us now what you know before the subpoenas arrived." Several of the people GSK attorneys contacted were never, ever going to receive subpoenas but as part of my deposition, GSK wanted to know who were our closest friends and who were we with the weekend before Stewart died. What also was interesting is that GSK attorneys called my friends on their cellphones rather than their landlines. I never gave out any numbers. I don't know for sure, but I think perhaps GSK's attorneys naively thought they would somehow catch my friends off guard and get more info.
Interesting.
Thank you. The word akathisa is relatively unknown to many. Can you tell me when you first heard the word and how it related to Stewart's death?
After Stewart, died nothing made sense. On Friday, August 13th a friend called me and said,
"akathisia killed Stewart." And of course, I replied,
"What?" She suspected early on that she thought Stewart's death was related to Paxil since that was the only thing that was different in his life that week. When I first heard the word, akathisia, I was walking my dog at the time. When I got home and wrote the word down, I decided to google "akathisia, Paxil, and suicide." All of a sudden this wealth of information appeared. One of the first articles that appeared was one by Dr. Peter Breggin titled "
How GlaxoSmithKline Suppressed Data on Paxil-Induced Akathisia: Implications for Suicidality and Violence." Then another article showed a connection between SSRIs and suicide and violence and included a definition of akathisia. It listed characteristics of akathisia. For the first time, what didn't make sense now became perfectly clear. Stewart's physical inner and outer restlessness, agitation and anxiety that I observed his last week of life now made sense. It was in this article that I first had the revelation that the drug I thought Stewart was ingesting to deal with his work related stress and anxiety instead created suicidal thoughts and actions, both of which he did not have previously.
The
article went on to state that Akathisia is so terrible,
"Death Can Be A Welcome Result." This is an actual quote by Dr. Roger Lane, the chief medical officer for Pfizer. Pfizer makes Zoloft, which like Paxil, is also a SSRI. It was at that moment I knew I needed to do something to help protect others and improve public health. How can this devastating drug side effect not be unknown to most health care professionals or patients?
After learning about akathisia, did you research attorneys who might help you seek justice?
I was told that Baum Hedlund was the best law firm in the country regarding pharmaceutical litigation. I was told very early on by Baum Hedlund that the generic issue would be a large hurdle.
Moving on to MISSD. Can you tell me what MISSD is all about and why it was important to create this organisation?
When Stewart died, I wanted to start an organization to raise awareness regarding akathisia. It is incredible how the organization name came to me. So many people were saying to me how awful it is when someone dies so young and how much they will miss their loved one. I kept hearing the name
"miss." That's how the name MISSD came to me. It stands for The Medication Induced Suicide Prevention and Education Foundation in Memory of Stewart Dolin.
After choosing the name, I gathered together close friends and family and was privileged to have the incredible Kim Witzcak as a board advisor. I have the best and most dedicated board. Since akathisia is what killed Stewart and very few people had ever heard of it, including health care providers, we decided our mission would be to educate the public regarding akathisia. The mission of MISSD is simple: To educate the public that when starting, stopping or changing a dosage of a medication like SSRI's, the drug side effect akathisia can occur. MISSD highlights the symptoms of akathisia and what to do if you are experiencing akathisia. We are a non-profit organization and take no money from pharmaceutical companies. This is important to note because many nonprofits do take money from the pharmaceutical companies and I believe this can create an unethical relationship.
MISSD presents at local, national and international conferences. We have created a booth and have exhibited in conference halls at too numerous to count. Last year our organization created an animated video about akathisia which has received almost 15,000 hits. In addition to the educational booth, pamphlets have been produced in English and Spanish that communicate the warning signs of akathisia, and we also have power point presentations. Two months ago my incredible board members and I presented at Loyola University Graduate School of Social Work.
MISSD is obviously near and dear to my heart. MISSD has saved lives and provided comfort to many people who have experienced such terrible loss. We have a "Share" link on our MISSD website, and I keep seeing similar stories posted over and over again. They always start out
"My loved one was fine, and then, gone, out of the blue, with no explanation." In the middle of the trial, a woman texted me stating her husband ended his life after starting Paxil. I believe he was prescribed Paxil not for depression, but in an attempt to deal with side effects from chemotherapy. As I keep hearing these real stories, it makes me more determined to spread the word of MISSD.
We are particularly interested in working with military groups given that the military suicide rate is at a record high. MISSD believes there is a correlation between the number of drug cocktails our veterans are prescribed and the increases in suicide and suicidality. In March, MISSD helped sponsor an event called
"K9's for Veterans" where I talked to more than 400 military vets and their family and friends regarding akathisia. After I had spoken, so many people came up to me and said thank you. They said,
"that happened to me" or
"It happened to someone I know." MISSD is important to my board and me because it is helping prevent needless deaths. We are all so proud and thankful for our supporters who have helped us make a positive impact. I believe MISSD is the first organization in the world to raise awareness about akathisia. We are a safe patient advocacy group. When we all realized Stewart's death could have been prevented, MISSD was our way to take action. Our knowledge of akathisia became a defining moment in all of our lives. We had to share this side effect so that the public can be better informed than we were.
What sort of response have you had from the launch of MISSD, have you come across any opposition from regulators or pharmaceutical companies?
No opposition from any regulators. At one point in my lawsuit, GSK wanted information on my board members, donators and GSK attorneys (either Andy Bayman or Todd Davis) presented print outs from our MISSD website. They wanted MISSD to be explored. Judge Zagel promptly stated MISSD was out of the lawsuit. The fact that GSK was worried about MISSD was gratifying because it confirmed we were shedding light on a subject they preferred to keep hidden.
The recently released MISSD video surely helps spotlight akathisia. What has been the overall response from the video?
Fantastic. We realized that if we were going to present to schools, hospitals, etc., we needed a powerful educational tool. We wanted a tool that was simple, short, and to the point. The video is creative and state of the art. Wherever we show the video, it is always very well received. It has been incredibly gratifying how well we have been received by the public. I think this is due in part because MISSD is not anti-drug, it is simply dedicated to raising awareness of akathsia and saving lives. Our mission resonates with so many people. Everything MISSD does is done very professionally, and we are viewed as a very important safe patient organization. Our initial fundraiser was primarily attended by friends, family members and associates of our board members. This is no longer the case. Today MISSD events are well attended, and I meet many new people for the first time at every event. They explain that they first found MISSD online as an important resource after their loved one died from prescription drug-induced akathisia. The families of akathisia victims who attend MISSD events come from all backgrounds and all parts of the country. We usually have more than 300 people at each event.
You've had many people visit Chicago from across the world, some of them also have tragic stories regarding the loss of loved ones due to prescription drug-induced akathisia. When did you realise the extent of this problem?
When Kim Witczak presented the Selling Sickness conference in Washington, D.C. in 2013, I met many people, such as Mathy Downing and Sara Bostock, who lost loved ones to akathisia. This was important as I started to realize I was certainly not alone.
Later when I spoke in Copenhagen with Kim and Mathy and met Steffini Lynch and Leonie Donnelly, it further emphasized this was a universal problem. Recently as the MISSD presence has expanded, I realize that through our website many people have come to Chicago to MISSD events and found comfort and support from the mission of MISSD.
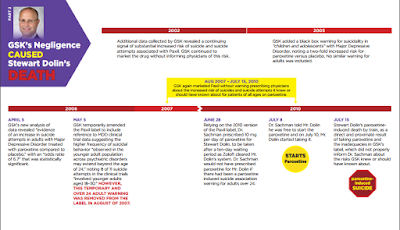
The jury unanimously agreed that GlaxoSmithKline is liable for not updating the Paxil label regarding the increased suicide risk created when adults take Paxil. In essence, the jury stated they believe, after hearing all the evidence presented by both sides, that Paxil caused Stewart's suffering and death. Furthermore, the jury believes GlaxoSmithKline knew about these potential risks yet failed to warn consumers.
During the trial it came to light that 22 patients died in Paxil clinical trials, 20 of these died by suicide, and the other two deaths are suspected to be suicides. All 22 victims were taking Paxil at the time, and 80% of these patients were over the age of 30. GSK likes to argue that it was an "illness" that caused these deaths and not Paxil. What would you say to the surviving family members of these clinical trial victims if you had a chance to meet them?
That is a great question because it brings up so many issues. GSK talks at length about underlying illness. Yes, there are people that kill themselves because they have had a lifelong history of mental health issues. They struggle and medications have been life-saving in many situations. However, when you talk to love ones of people who died from akathisia you hear from many of them that the drugs were prescribed for issues such as insomnia, test anxiety, or situational stress. The drug companies seem to want to pathologize what it means just to be human.
During the trial, my sister sat through opening arguments and texted me,
"I don't know who they are talking about." GSK tried to create a view of Stewart that quite frankly didn't exist. But specifically, regarding prescription drug-induced suicides, I would tell the surviving family members to realize the death was not the fault of their loved one. People sometimes say that when someone ends their life, it was their choice. I am not sure that that is a correct statement either. But death by akathisia is not a choice. It is not a suicide. It is a fatal drug reaction.
Additionally, I would tell surviving family members to get involved. There is a favorite quote of mine from the anthropologist Margaret Mead,
"Never doubt that a small group of thoughtful, committed citizens can change the world; indeed, it's the only thing that ever has." Talk to others, spread the word regarding akathisia, contact government agencies. As we learned from this lawsuit, GSK blamed the FDA; We need to be proactive and contact our regulatory agencies to say inadequate warnings are just not acceptable. What this lawsuit has shown is that akathisia is a real, legitimate adverse drug reaction. The public needs to be aware of akathisia signs and symptoms.
Do you have any advice for consumers who are considering pharmaceutical industry litigation?
I think the person has to be aware that this process is emotionally and physically difficult. In addition to having the necessary courage and conviction, it is imperative to have top lawyers. My lawyers from Baum Hedlund and David Rapoport were incredible. They are professional and highly knowledgable. But they are also amazing human beings who understand the injustice that was done to Stewart. Our work together felt less like a lawsuit and more like a personal journey and commitment shared by all of us.
We know Glaxo is appealing the verdict. This means the funds the jury award for Stewart's avoidable death and suffering will be held until the appeal process is finished. There have been a few online comments left on media articles in which a few posters have suggested this trial is just about money. How do you respond to people who suggest such?
I always want to respect people's divergent opinions, and I can understand from the outside looking in one interpretation of the lawsuit might be that is about money. However, this notion is furthest from the truth. I would hope these individuals actually understood what critical information was highlighted in this trial because this information affects their lives as well as Stewart's. I hope people would educate themselves regarding drug safety, drug studies, the role of the FDA, generics, etc. The vast majority of people have had very, very positive reactions to the verdict. There will always be people who disagree, and that is their prerogative.
I knew from the moment this lawsuit was filed that GSK was always concerned that this was a generic drug. I was told before we even went to trial, that, if GSK lost, they would appeal. In fact, I believe there was a lawyer in the courtroom for GSK that was there for the sole purpose of gathering information to start the appeal process. Appeals take several years and, of course, I could lose on appeal. It has been suggested that GSK wants to take this case to the Supreme Court because they are so afraid of what this guilty verdict means. As it stands, the legal ramifications for this verdict are so damaging for pharmaceutical companies that reaching the Supreme Court is very possible. That could take 5-7 years.
Clearly this case has never been about money. For me, it has always been about awareness, highlighting akathisia and ultimately changing the black box warning to include all ages. If those individuals who think this case is about money actually read the entire articles, they would learn about MISSD and all the work I do to increase akathisia awareness. While I am eternally grateful to the generosity of our supporters, I have also used my own resources to help educate the public about akathisia. I do this in honor of Stewart and to help others avoid similar tragedy.
Thank you, Wendy.
Please, if there is anything you want to say to the readers of my blog, feel free to do so.
I am so grateful to the overwhelming support of many people from all over the world. A special thanks to you, Bob, for all you have done over the past years to raise awareness of drug side effects, specifically akathisia, and my lawsuit. You have devoted so much of your time and resources to this case, and I am eternally honored by your efforts. You are remarkable. Thank you so much.
Links
What is Akathisia? (Short Educational Video)
MISSD
Baum, Hedlund, Aristei & Goldman
Rapoport Law Offices, P.C
Dolin Vs GSK Paxil Trial Court Transcripts
Dolin v. GSK Paxil Trial Exhibits
--
Dolin Vs GSK Blog Coverage
Dolin v GSK - Opening Arguments
Dolin Vs GSK - Day Two - "Jack-In-The-Box"
Dolin vs GSK - Healy 'Rocks Da House'
Dolin Vs GSK - JP Garnier Video Deposition
Dolin Vs GSK - The Dunbar Tape
Dolin Vs GSK - Day 4 - Slam Dunk
Dolin Vs GSK - 8.9 Suicide Increase For Adult Paxil Users
Dolin Vs GSK - Day 6 - Ass Kicking Semantics
Dolin Vs GSK - Day 7 - Abraham Lincoln
Dolin Vs GSK - Day 8 - Get to the Point, Todd!
Dolin Vs GSK - Glenmullen Nails It!
Dolin Vs GSK - "Babes"
Dolin Vs GSK - Wendy's Cross and GSK's Petition
Dolin Vs GSK - Robert "Bling Bling" Gibbons
Dolin Vs GSK: Suicide Prevention Warning "Futile", Claims GSK Exec
Dolin Vs GSK : Jury shown List of the Dead in Paxil Clinical Trials
Dolin Vs GSK: Last Man Standing & The Return of Dr. Healy
Dolin Vs GSK: Closing Arguments
Dolin Vs GSK - The Verdict
--