"It's not about what they tell you, it's about what they don't."
~ Bob Fiddaman, Author, Blogger, Researcher, Recipient of two Human Rights awards
Sunday, August 31, 2008
The Risks Outweigh the Benefits - An Interview with Bob Fiddaman
Here is the result of that interview.
----
Robert Fiddaman(1), Gary L. Hart(2), Michelle Hart(3), Matthew Holford(4)
Foreword
As doctors, we sell our services under the banner of science. Many patients have begun to question the quality, transparency and honesty of our science. Unfortunately, they are doing so with good reason.
When our patients lose trust in the integrity of the science we may never be able to recover our status as a profession. We ignore questioning patients at our peril.
Bob Fiddaman is a question-asker. He is persistent and he is angry. He is also right.
We need to start listening.
Aubrey Blumsohn
MBBCh, PhD, MSc, BSc(hons), FRCPath
Read the new book, The Evidence, However, Is Clear...The Seroxat Scandal
By Bob Fiddaman
ISBN: 978-1-84991-120-7
CHIPMUNKA PUBLISHING
AVAILABLE FOR DOWNLOAD HERE
PAPERBACK COMING SOON
Saturday, August 30, 2008
Just seems this Harassment won't stop!
Read the new book, The Evidence, However, Is Clear...The Seroxat Scandal
By Bob Fiddaman
ISBN: 978-1-84991-120-7
CHIPMUNKA PUBLISHING
AVAILABLE FOR DOWNLOAD HERE
PAPERBACK COMING SOON
Here you will find stories about Paxil and Paxil withdrawal from around the world
Here you will find stories about Paxil and Paxil withdrawal from around the world. These articles have been selected to match the general theme of this site and are meant to support its general thesis, namely that paroxetine withdrawal is a real phenomenon. To this end, only articles from established news sources have been include. At the bottom of the page, you will find the latest news headlines surrounding Paxil and GSK. This stories are automatically posted and are not edited for content.
Antidepressant Seroxat tops table of drug withdrawal symptoms
Breakup with Paxil hard to do
Le difficile sevrage au Paxil (In French)
Withdraw slowly from pills that affect the brain
Paxil Five-Year Litigation History
Drip Drip Drip - Paxil Info Leaks Out
Antidepressants in Pregnancy Linked to Newborn Hangover
The 10 Worst Corporations of 2004
Drug Maker Withheld Paxil Study Data
Revealed: secret plan to push'happy' pill
FDA bolsters anti-depressant warning
Anti-Depressant Creates Conflicts For Patients
Why millions of women are hooked on the happy pills
Young people advised not to use Seroxat
Seroxat: E-mails from the edge
Seroxat maker abandons 'no addiction' claim
Paxil And Addiction
The Secrets of Seroxat
'Pushing' Pills to the Anxious
Suit filed against anti-depressant Paxil
Here, you will find a cross-section of the thousands of letters that *I have received over the years. They are meant to serve as examples of the type of correspondence, received and will ,hopefully, mirror the variety of visitors that view these pages every day. Preference is given to shorter, well written letters, although neither are necessary attribute. Letters are not edited in any way, except to remove names and e-mail addresses.
*I - refers to the author of QuitPaxil.org
Read the new book, The Evidence, However, Is Clear...The Seroxat Scandal
By Bob Fiddaman
ISBN: 978-1-84991-120-7
CHIPMUNKA PUBLISHING
AVAILABLE FOR DOWNLOAD HERE
PAPERBACK COMING SOON
Paxil Resource Web Site Launched by Williams Kherkher
Houston, TX (PRWEB) June 26, 2007 -- In response to recent news regarding the prescription drug Paxil, Williams Kherkher of Houston, Texas has launched a complete Web site, whose URL is Paxil.
Williams Kherkher is led by Attorneys John Eddie Williams and Steve Kherkerk, and has 26 attorneys and over 120 support staff and has worked in the area of mass tort litigation since 1983.
Paxil is manufactured by SmithKline Beecham, and is part of the same drug "family" as Prozac, Zoloft, Luvox and Celexa, all of which are considered "SSRI's," which stands for Selective Serotonin Reuptake Inhibitors. Paxil's primary use is to help treat depression. Several lawsuits have resulted from the discovery of these problems, and one class action lawsuit, Hoormann v. SmithKlineBeecham, Case No.04-L-715 in the Third Judicial Circuit for the State of Illinois, recently settled for $63.8 million.
As early as 2005, the Food and Drug Administration (FDA) published a warning on its Web site stating that Paxil was linked to the following side effects:
birth defects
restlessness
hallucinations
loss of coordination
fast heart beat
increased body temperature
fast changes in blood pressure
overactive reflexes
diarrhea
coma
nausea
vomiting
Paxil has also been found to be addictive, and there have even been allegations that the company suppressed data that showed Paxil to be a high risk for children and adolescents.
In regards to the birth defects link, the women who are most at risk are those in their first three months of pregnancy, and the specific birth defect that's been found to be most prevalent is a heart defect, but the FDA also warned that no one should simply stop taking the drug without the involvement of their medical doctors.
Read the new book, The Evidence, However, Is Clear...The Seroxat Scandal
By Bob Fiddaman
ISBN: 978-1-84991-120-7
CHIPMUNKA PUBLISHING
AVAILABLE FOR DOWNLOAD HERE
PAPERBACK COMING SOON
Withdrawal Symptoms From Paroxetine May Last For A Long Time
When withdrawal symptoms ensue after discontinuation of a drug, such as paroxetine, it is generally assumed they will last for a few weeks and then they will subside. Many patients do not think so and some of them have also created a website concerned with paroxetine discontinuation effects.
Now researchers at the University of Bologna headed by Prof. G.A. Fava confirm their account in an article published in the December 2007 issue of the International Journal of Neuropsychopharmacology.
The aim of this investigation was to explore the prevalence and features of discontinuation syndromes ensuing with gradual tapering of selective serotonin reuptake inhibitors (SSRIs), in optimal clinical conditions in patients with panic disorder and agoraphobia. Twenty-six consecutive outpatients met the DSM-IV criteria for panic disorder and agoraphobia while taking SSRIs. Twenty remitted upon behavioural treatment. Antidepressant drugs were then tapered at the slowest possible pace and with appropriate patient education. Patients were assessed with the Discontinuation-Emergent Signs and Symptoms (DESS) checklist 2 wk, 1 month and 1 yr after discontinuation. Nine of the 20 patients (45%) experienced a discontinuation syndrome, which subsided within a month in all but three patients who had been taking paroxetine for a long time. Discontinuation syndromes appeared to be fairly common even when performed with slow tapering and during clinical remission. In some cases disturbances persisted for months after discontinuation.
PSYCHOTHERAPY AND PSYCHOSOMATICS
http://www.karger.com/pps
Read the new book, The Evidence, However, Is Clear...The Seroxat Scandal
By Bob Fiddaman
ISBN: 978-1-84991-120-7
CHIPMUNKA PUBLISHING
AVAILABLE FOR DOWNLOAD HERE
PAPERBACK COMING SOON
Glaxo Loses Preemption Ruling In Paxil Suicide
On September 14, 2002, 16-year-old Jake Garrison shot himself to death, eight months after first being prescribed the Paxil antidepressant. And so a lawsuit was filed against Glaxo, claiming the drugmaker knew there were risks associated with off-label use pediatric use of Paxil and, therefore, had a duty to warn of those risks.
However, Glaxo cited preemption as a reason for the suit to be dismissed. Preemption is the legal notion that FDA approval of a drug supercedes state law claims challenging safety, efficacy, or labeling. Drugmakers and the FDA argue that preemption exists by maintaining the agency’s actions are the final word on safety and effectiveness.
In this case, the drugmaker maintained that a direct conflict existed between failure-to-warn claims under New Jersey state law and the federal Food, Drug & Cosmetic Act. New Jersey law stipulates that a drugmaker must warn of all known adverse effects of a drug as soon as reasonably feasible after learning of any danger. Lawyers for the Garrison family rejected the argument in their motion.
However, Glaxo contended that, had it included warnings about an increased risk of pediatric suicide in the Paxil label, the pill would have been considered misbranded under federal law. And since Glaxo could have not remained in compliance with both state and federal law as it stood prior to September 2002, when Garrison committed suicide, Glaxo argued a conflict existed, triggering preemption of New Jersey law.
But Judge Ronald Buckwalter of the US District Court for the Eastern District of Pennsylvania rejected the argument by ruling FDA regs allow a drugmaker to “unilaterally add a warning on a drug, so long as the drug manufacturer has reasonable evidence of an association of a serious hazard with a drug.” Here is his 53-page ruling, in which he also wrote:
“Arguably, Glaxo possessed such reasonable evidence of an association between Paxil and pediatric suicidality as early as 1998, at the conclusion of their own Studies 329 and 377, the data from which initially triggered the FDA’s concerns.
“The mere fact that Glaxo elected not to submit the results of those studies until April of 2002, when it filed a supplemental NDA seeking approval of an indication for pediatric use, does not detract from the fact that it may have had reasonable evidence of a hazard prior to that time…The mere fact that the FDA had not ordered Glaxo to include this warning prior to 2002, does not mean that it could not have legally done so.”
Preemption is a hot legal topic these days. The US Supreme Court will review a case on November 3, and if you have some time on your hands, open this link to you will find background material and numerous briefs backing and opposing preemption.
By the way, this is the second Paxil case this summer in which preemption was rejected as a defense. This is the other one.
Read the new book, The Evidence, However, Is Clear...The Seroxat Scandal
By Bob Fiddaman
ISBN: 978-1-84991-120-7
CHIPMUNKA PUBLISHING
AVAILABLE FOR DOWNLOAD HERE
PAPERBACK COMING SOON
Mail Order Academics
Read the new book, The Evidence, However, Is Clear...The Seroxat Scandal
By Bob Fiddaman
ISBN: 978-1-84991-120-7
CHIPMUNKA PUBLISHING
AVAILABLE FOR DOWNLOAD HERE
PAPERBACK COMING SOON
Friday, August 29, 2008
Dependence on Paroxetine (Paxil/Seroxat)
Statement by David Healy MD FRCPsych
I have been asked to confirm and comment on evidence relating to withdrawal reactions suggestive of physical dependence on paroxetine, observed in studies on healthy volunteers, carried out in the 1980s by SmithKline Beecham.
In the course of a recently settled legal action (Tobin v SmithKline, Wyoming, 2001), I acted as an expert witness for the Plaintiffs. As part of the discovery process I had sought and was granted access to SmithKline’s Beecham’s healthy volunteer archive at Harlow. My concern had been to scrutinise those records for details of possible agitation and suicidality in healthy volunteers taking paroxetine. These were present, but at least as striking was evidence from these studies about dependence on paroxetine.
A detailed expert report was prepared for the plaintiffs’ lawyers in this case, which includes details of studies undertaken by SmithKline Beecham that fully substantiate concerns I communicated to the UK Medicines Control Agency in letters of 7th and 19th June 2001, the essence of which was also accurately reported in The Guardian newspaper (11 June 2001)
I regret that I am under a confidentiality order in regard to this material and am not able to disclose it to this appeal. However, I can confirm, and am prepared to testify to the substance of the points raised in the following exchange (in my testimony in Tobin v SmithKline) between Mr Charles Preuss, the attorney for SmithKline, and myself.
Healy: Yes, but there's a withdrawal syndrome from Paxil, including agitation, abnormal dreams and nightmares that comes through in spades in these healthy volunteer studies.
Preuss: You're saying Paxil is still active for three months?
Healy: In up to 80 percent of the volunteers on this drug for only two weeks produces withdrawal syndromes in these healthy volunteers. I'm saying in my clinical experience I've seen people on this drug for short periods of time and I've seen them have troubles three months later, yes.
My concerns about paroxetine extend far beyond the results of these studies on healthy volunteers. In the 1990s, after its release on to the market as an antidepressant, SmithKline Beecham put paroxetine into clinical trials – exemplified by the study reported by Montgomery & Dunbar, 1993 - that involved a randomised discontinuation design. The difficulties experienced by patients on randomisation to placebo were then interpreted by SmithKline Beecham as evidence of new illness episodes, and the company has subsequently responded to enquiries about the risk of withdrawal reactions and physical dependency, typically by stating that any such problems experienced by patients are simply a recrudescence of their original nervous problem. Basic pharmacological principles, epidemiological studies on depression, as well the evidence from their own healthy volunteer studies strongly suggest that such an interpretation of these data was and is quite unjustified.
Against this background SmithKline Beecham launched paroxetine in the UK with disclaimers on the datasheet to the effect that, as with any drug acting on the brain, some care needs to be taken on discontinuation. The data available to SmithKline before launch indicated problems occurring at a significantly greater rate and to a markedly more severe degree than any psychiatrist at the time would have had reason to expect either from an antidepressant or from such warnings.
Post-marketing surveillance surveys and other studies undertaken since have indicated much greater withdrawal problems with paroxetine than with the previous generation of tricyclic, MAOI and non-tricyclic or non-MAOI antidepressant drugs. A randomised controlled trial undertaken with funding provided by Eli Lilly (Rosenbaum et al, 1998), indicated rates of problems on discontinuation of paroxetine in over 30% of patients with many patients having multiple symptoms, including many novel and disturbing symptoms.
For Dr Wheadon and the company therefore to characterise paroxetine withdrawal reactions as very rare, transient, mild and/or virtually impossible to detect and distinguish from underlying psychiatric illness is simply an untenable position. It follows that I have real concerns about SmithKline promoting paroxetine for the prophylaxis of depressive disorders and other psychiatric illness, on the basis of data that are more sensibly and credibly explained in terms of physical dependence and withdrawal symptoms.
David Healy MD FRCPsych.
Read the new book, The Evidence, However, Is Clear...The Seroxat Scandal
By Bob Fiddaman
ISBN: 978-1-84991-120-7
CHIPMUNKA PUBLISHING
AVAILABLE FOR DOWNLOAD HERE
PAPERBACK COMING SOON
Seroxat/Paxil Journal - The Video
Read the new book, The Evidence, However, Is Clear...The Seroxat Scandal
By Bob Fiddaman
ISBN: 978-1-84991-120-7
CHIPMUNKA PUBLISHING
AVAILABLE FOR DOWNLOAD HERE
PAPERBACK COMING SOON
Thursday, August 28, 2008
The Video GSK Wanted Banned
This one goes out to UK Survivors... Particularly Jeremy and 'Tuesday' - His alter ego
Read More in The Evidence, However, Is Clear...The Seroxat Scandal [The Ebook]
Chipmunka Publishing ISBN: 978-1-84991-120-7
Paperback coming soon
Good Friend of Mine
Took me 7 hours to create this video.
Read the new book, The Evidence, However, Is Clear...The Seroxat Scandal
By Bob Fiddaman
ISBN: 978-1-84991-120-7
CHIPMUNKA PUBLISHING
AVAILABLE FOR DOWNLOAD HERE
PAPERBACK COMING SOON
Antidepressant Seroxat tops table of drug withdrawal symptoms
The committee on the safety of medicines, which receives reports of drug side-effects from doctors and pharmacists, has received an avalanche of complaints about Seroxat, one of the class of drugs known as SSRIs (selective serotonin reuptake inhibitors). The SSRIs, including Prozac, have always been marketed as safe medicines which are supposed not to cause the dependence problems that emerged with older drugs such as Valium and Ativan.
Seroxat - known generically as paroxetine - leads the top 20 table of drugs causing withdrawal problems, with 1,281 complaints from doctors under the "yellow card" scheme set up for the reporting of medicines' side-effects. More reports have been filed about Seroxat than about the rest of the top 20 put together. In the top six, five of the drugs said to be causing withdrawal problems are SSRIs - second after Seroxat comes Efexor (venlafaxine), with 272 complaints.
Read More
Read the new book, The Evidence, However, Is Clear...The Seroxat Scandal
By Bob Fiddaman
ISBN: 978-1-84991-120-7
CHIPMUNKA PUBLISHING
AVAILABLE FOR DOWNLOAD HERE
PAPERBACK COMING SOON
Over 500 complain of side-effects from GSK drug
Hat - Tip - The Truthman
Over 500 people have complained of side effects from the reformulation of a commonly used drug, prompting calls for alternatives to be made available.
In June health authorities announced they were urgently investigating problems with Eltroxin, a hyporthyroidism drug taken by 70,000 New Zealanders.
Their action followed a rash of reports of nausea, headaches and weight gain, since the drug was reformulated by manufacturer GlaxoSmithKline (GSK) over a year ago.
Read More Here
Read the new book, The Evidence, However, Is Clear...The Seroxat Scandal
By Bob Fiddaman
ISBN: 978-1-84991-120-7
CHIPMUNKA PUBLISHING
AVAILABLE FOR DOWNLOAD HERE
PAPERBACK COMING SOON
IT'S HERE
Read the new book, The Evidence, However, Is Clear...The Seroxat Scandal
By Bob Fiddaman
ISBN: 978-1-84991-120-7
CHIPMUNKA PUBLISHING
AVAILABLE FOR DOWNLOAD HERE
PAPERBACK COMING SOON
Wednesday, August 27, 2008
It's Nearly Here!
Train won't be stopping.
Read the new book, The Evidence, However, Is Clear...The Seroxat Scandal
By Bob Fiddaman
ISBN: 978-1-84991-120-7
CHIPMUNKA PUBLISHING
AVAILABLE FOR DOWNLOAD HERE
PAPERBACK COMING SOON
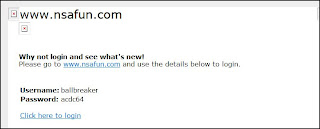
HARASSMENT STATEMENT
August 27 2008
By Bob Fiddaman
This is a statement regarding the person who has been harassing me for sometime now. He is a regular poster on UK Survivors. To have your name associated with a known pedophile, to be labeled a benefit cheat and racist on a public forum and to also have images of myself stuck on a cartoon then labeled a 'Date Drug Rapist' is downright disgusting. This sad individual has now created a blog about me. He has now posted my address on this new blog he has created. So what do I tell my children when they come look after my place when I am not here?
A few people on UK Survivors have joined in with his harassment of me... they are as guilty of abuse as what he is.
I actually fear for my safety now because this individual has made some vile accusations about me and by posting my address online he has possibly made me and my children the target of low life's out there. He has also now started targeting friends of mine and posting on various forums throughout cyberspace.
Posts by this man on UK Survivors that relate to me have been logged.
He still continues to post on his new blog and in UK Survivors.
He claims that I liked a parrot sketch with my image on top - he is mistaken. The actual post made by someone else in UK Survivors, that I did like was of two parrots with an image of my face on them. I saw no harm in it.
The image I did not like was of a parrot with my image as the head that was preceeded with the headline 'Evidence Proves Date Drug Rapist Pirate Bob Molested Parrots' - This message has since been removed from UK Survivors. The person in question either removed it because he knew it was defamatory, The Group Moderator either removed it or Yahoo Groups moved it.
The individual continues to post about me on his new blog and on UK Survivors.
Both West Midlands Police and the Police Service of Northern Ireland [PSNI] have been informed.
Statement Ends.
Bob Fiddaman
Author of Seroxat Sufferers Blog
Read the new book, The Evidence, However, Is Clear...The Seroxat Scandal
By Bob Fiddaman
ISBN: 978-1-84991-120-7
CHIPMUNKA PUBLISHING
AVAILABLE FOR DOWNLOAD HERE
PAPERBACK COMING SOON
Monday, August 25, 2008
Patient Advocate in Harassment Campaign
This blog is my stand. I believe GlaxoSmithKline are guilty of selling a defective drug and are guilty of hiding and/or manipulating the data for that drug.
I've been banging the drum loudly from my little place in Birmingham, others have joined in the campaigning, lobbying MP's or starting up their own blogs to help spread the word.
I believe this 'banging of the drum' has got me my meeting with Kent Woods next month at MHRA Headquarters.
I certainly expected critiques of many of my rants on here, for one, I didn't expect GlaxoSmithKline to take what shit I had to throw at them lying down. They regularly visit this blog [I have the stats].
What I didn't expect was a fellow campaigner, who originally had a gripe with GlaxoSmithKline, to post remarks about me on a mental health forum.
This week I took the unprecedented measure of joining this forum. I openly asked for this person to ask me questions and I would answer them to the best of my ability providing he would answer my questions for his readers. I stayed in the group for 24 hours. He never answered any of my questions, instead he posted vile comments about me. Associating me with a well-know pedophile, labeling me a benefit cheat and a racist. One of his members saw fit to add an image of my face to a cartoon and label me a serial rapist!
Trawling through the archives of the forum I found many comments left by this individual and in the 'Files' section of the group found three photo's of me for his members. When you read remarks such as the ones left by this individual it kind of turns your stomach. Further research through the archives show this person openly admitting that he was once arrested at gunpoint by the Irish Police. That kinda scared me. If he can break restraining orders and scare his wife so much that she calls the police then he could possibly have some serious mental health issues.
To this end, I contacted Yahoo Groups Complaints Department - 5 of the offending posts have now been removed, whether he has moved them, the group owner has moved them of Yahoo Groups have moved them is unknown.
I used to have a myspace and facebook account - these were removed through fear of this very sad individual 'involving' my friends with his bile. In fact he recently uploaded a picture of one of my friends to his little forum.
I find it utterly baffling why a fellow campaigner for better drug regulations in the UK would target not only me but two other Seroxat campaigners. It is plainly obvious that he cannot enter debate, he had 24 hours in which to do so but decided to post old articles about me instead.
Strangely, in 2003 he posted regularly about Seroxat and GlaxoSmithKline, he even taunted Alistair Benbow in some of his posts but somewhere along the line his opinion was changed, instead of targeting GSK and Benbow, he now targets myself and other Seroxat campaigners.
In June this year he wrote to Solicitors handling the Seroxat litigation demanding to know how I could afford a trip to Australia many years ago, he also asked why in the one photo he has of me in Australia, I look so 'larger than life', assuming that I was suffering Seroxat withdrawal at the time the photo was taken.
It has become an obsession for him to trawl through Internet archives and post about me, targeting a poetry site where I used to post then labeling me a 'Tampon Poet'. Yeh, I wrote poetry years ago, yeh I wrote one about Tampons, it's called humour.
He also could not answer whether he had wrote to a mental health charity CEO offering him the same snaps of me in Australia.
I know he wrote to my M.P Gisela Stuart with photos and various claims.
I can put up with critiques of my writing on here, I can even hold my hand up and say I have made a mistake, if it is pointed out to me.
One thing I cannot.. and never will put up with is claims such as 'Benefit Cheat', 'Racist' and associating me with a well known sexual molester of children. The individual wrote " Bangkok said no to Garry Glitter ....but no probs for Fiddaman" - He then posted a photo of me standing in the streets of Bangkok.
Some of his posts were entitled:
I wonder what Scientology lover Bob Fiddaman makes of this
Benefit cheat Fiddaman off to see Kent Woods @ MHRA
Fiddaman making dodgy remarks about Muslims !!
and in the files section he had three photos of me (one taken with a woman)
Two of them were entitled
1. Benefit cheat Bob Fiddaman in Bangkok
2. Benefit cheat Bob Fiddaman in Melbourne
Another member writes... "Evidence Proves Date Drug Rapist Pirate Bob Molested Parrots"
It's a shame that he once used to be focused and belittle Seroxat, he even defended a fellow campaigner whom he now 'slags' off, by writing on a Channel Four Forum. He was incensed that a Seroxat victim had been treated unfairly on the Richard & Judy Show here in the UK. He now 'bad mouths' this Seroxat victim.
I feel for this individual, he needs mental help, hence the reason why I have refrained from naming him in this post.
Yahoo Groups Complaint Dept are investigating further posts he has written and earlier today I contacted West Midlands Police to investigate my claim of Harassment from this individual. No doubt they will be in touch over the coming days.
The jury is out to see whether this very sad individual has learned his lesson.
Read more about this insidious coward HERE
Fid
Read the new book, The Evidence, However, Is Clear...The Seroxat Scandal
By Bob Fiddaman
ISBN: 978-1-84991-120-7
CHIPMUNKA PUBLISHING
AVAILABLE FOR DOWNLOAD HERE
PAPERBACK COMING SOON
Glaxo HIV Ads Use Scare Tactics?
AIDS activists say the drugmaker’s magazine ads in the US are nothing more than thinly disguised attempts to scare patients away from trying new drug regimens, The Wall Street Journal reports.
Bob Huff, antiretroviral project director at Treatment Action Group, an advocacy group in New York, tells the paper he complained to Glaxo a few months ago about an ad that shows shark-infested waters with the message: “Don’t take a chance - stick with the HIV medicine that’s working for you.” Huff calls the ad offensive and aimed at instilling fear in patients. The ads, by the way, carry Glaxo’s logo but don’t promote specific drugs.
Read more HERE
Read the new book, The Evidence, However, Is Clear...The Seroxat Scandal
By Bob Fiddaman
ISBN: 978-1-84991-120-7
CHIPMUNKA PUBLISHING
AVAILABLE FOR DOWNLOAD HERE
PAPERBACK COMING SOON
Saturday, August 23, 2008
‘The medication had to play a part in it’
BY: Stephen Crane
Many in this community are still in disbelief, struggling to understand what happened to one of their own, one of their sons. For weeks, people rode the rollercoaster. They awaited word of his whereabouts. They assisted in the search. And they were plagued with questions in the aftermath.
These are questions not easily answered – daunting questions without a simple reply. But Carole Richie has been asking them. Her son’s death demanded it.
“Anybody who knows Garrett knew that this was not Garrett,” Richie said. “And the medication had to play a part in it, because he was so outgoing and had his life planned out, everything planned out. And this is something that just went wrong real quickly, in a matter of hours.”
She found Garrett Bardin’s prescription bottle of Paxil while the search was still ongoing, and the discovery gave her pause even then.
Story continues HERE
Read the new book, The Evidence, However, Is Clear...The Seroxat Scandal
By Bob Fiddaman
ISBN: 978-1-84991-120-7
CHIPMUNKA PUBLISHING
AVAILABLE FOR DOWNLOAD HERE
PAPERBACK COMING SOON
Friday, August 22, 2008
30 Seconds of Happiness - AC/DC
This is a sneak preview [audio] of AC/DC's new single 'Rock N' Roll Train'
Read the new book, The Evidence, However, Is Clear...The Seroxat Scandal
By Bob Fiddaman
ISBN: 978-1-84991-120-7
CHIPMUNKA PUBLISHING
AVAILABLE FOR DOWNLOAD HERE
PAPERBACK COMING SOON
GSK to report organization grants
The latest effort to stifle bloggers, government officials and various media sources is nothing more than a publicity stunt.
We are already aware of the grants GSK have given to the likes of Keller and other Seroxat Gunslingers and also to 'experts' who 'big up' Seroxat.
I can just hear the spokesperson from GSK now... "We put all our information online that is readily available to the public."
Be nice if they put information on their website as to why they have made countless settlements out of court then placed gagging orders on those affected by Seroxat!
So next time GSK claim to be transparent, ask them why they place gagging orders on individuals they settle out of court with.
Fid
Read the new book, The Evidence, However, Is Clear...The Seroxat Scandal
By Bob Fiddaman
ISBN: 978-1-84991-120-7
CHIPMUNKA PUBLISHING
AVAILABLE FOR DOWNLOAD HERE
PAPERBACK COMING SOON
Thursday, August 21, 2008
More debate on GSK's Cervarix
Researchers Question Wide Use of HPV Vaccines
By ELISABETH ROSENTHAL
Published: August 20, 2008
Two vaccines against cervical cancer are being widely used without sufficient evidence about whether they are worth their high cost or even whether they will effectively stop women from getting the disease, two articles in this week’s New England Journal of Medicine conclude.
Both vaccines target the human papillomavirus, a common sexually transmitted virus that usually causes no symptoms and is cleared by the immune system, but which can in very rare cases become chronic and cause cervical cancer.
The two vaccines, Gardasil by Merck Sharp & Dohme and Cervarix by GlaxoSmithKline, target two strains of the virus that together cause an estimated 70 percent of cervical cancers. Gardasil also prevents infection with two other strains that cause some proportion of genital warts. Both vaccines have become quick best sellers since they were licensed two years ago in the United States and Europe, given to tens of millions of girls and women.
“Despite great expectations and promising results of clinical trials, we still lack sufficient evidence of an effective vaccine against cervical cancer,” Dr. Charlotte J. Haug, editor of The Journal of the Norwegian Medical Association, wrote in an editorial in Thursday’s issue of The New England Journal. “With so many essential questions still unanswered, there is good reason to be cautious.”
In her article, Dr. Haug points out the vaccines have been studied for a relatively short period — both were licensed in 2006 and have been studied in clinical trails for at most six and a half years. Researchers have not yet demonstrated how long the immunity will last, or whether eliminating some strains of cancer-causing virus will decrease the body’s natural immunity to other strains.
More to the point, because cervical cancer develops only after years of chronic infection with HPV, Dr. Haug said there was not yet absolute proof that protection against these two strains of the virus would ultimately reduce rates of cervical cancer — although in theory it should do so.
FULL STORY NYT NEW YORK TIMES
Related Links:
Body Count Linked to GSK Vaccine Trial Rises
UK babies given toxic vaccines, admits Glaxo
Drug Company Admits Unsafe Vaccines Were Used
The Bitter Feud over LYMErix
New York's HIV experiment
Read the new book, The Evidence, However, Is Clear...The Seroxat Scandal
By Bob Fiddaman
ISBN: 978-1-84991-120-7
CHIPMUNKA PUBLISHING
AVAILABLE FOR DOWNLOAD HERE
PAPERBACK COMING SOON
Wednesday, August 20, 2008
Cervical Cancer, Ofsted & GlaxoSmithKline
You may remember the uproar when Paul Blackburn was appointed to the board of Ofsted earlier this year. Blackburn is a senior vice-president at GlaxoSmithKline. His appointment came two weeks before the company won a reported £100million contract to vaccinate all schoolgirls of 12 and 13 against the sexually transmitted virus linked to cervical cancer.
Pressure from bloggers and other parties, I believe, forced the resignation of Blackburn and left Ofsted red faced.
Yesterday's news that the NHS will be treating 12 year old girls with Blackburn's employers vaccine should come as no surprise to us all.
With such an apparent tainted history regarding children and Glaxo's drugs, the NHS have simply turned a blind eye. To put it bluntly they are allowing a company with an apparent history of woeful neglect of our children in through the door to once again ply their wares.
If you are a parent with a 12 year old daughter, do your research. Take a look through the many stories where Glaxo have showed utter contempt for children.
If you ask me, Ofsted walked away from this debacle unscathed.
Why did they appoint Blackburn in the first place?
Schools Secretary Ed Balls, one of Gordon Brown’s closest advisers, said Mr Blackburn had a ‘passion’ for helping children.
So did Mr. Jones, my school teacher from many years ago!
Teresa Cooper, author of 'Pin Down' and No2Abuse also highlighted the Ofsted appointment of Blackburn. Yesterday, she was forced to resign from her job. She believes it was because she had highlighted this particular story on her website. She left her employers yesterday... her employers biggest customer? One GlaxoSmithKline! Read her story here
Hat tip: soulful sepulcher
Fid
Read the new book, The Evidence, However, Is Clear...The Seroxat Scandal
By Bob Fiddaman
ISBN: 978-1-84991-120-7
CHIPMUNKA PUBLISHING
AVAILABLE FOR DOWNLOAD HERE
PAPERBACK COMING SOON
16 Year Old Haley Stands Up To Be Counted
Haley has took the bull by the horns, she too is sticking her middle finger up to Pharma and the regulators. She too, like Manie, has been let down by the system.
She wrote me and said she would be honoured if I left a comment on her blog. Well, Haley, I am honoured to be in the thoughts of your family. Grit, determination, bravery and strong will are traits that will unite you all and keep you standing.
Keep up the good fight.
I salute you.
Fid
Haley's blog can be read here
Read the new book, The Evidence, However, Is Clear...The Seroxat Scandal
By Bob Fiddaman
ISBN: 978-1-84991-120-7
CHIPMUNKA PUBLISHING
AVAILABLE FOR DOWNLOAD HERE
PAPERBACK COMING SOON
Tuesday, August 19, 2008
How doctors are turning millions of us INTO addicts
How doctors are turning millions of us INTO addicts
By Jerome Burne
Source: Daily Mail
Gina Loxam was feeling a bit low, so she went to see her GP and was prescribed the anti-depressant, Seroxat.
Ten years later, she is still on the drug because the severe mood swings, headaches, fatigue and weight gain she suffers when she tries to come off are unbearable.
Gina, a 52-year-old finance and quality manager, is one of more than 600 people now suing the pharmaceutical company GlaxoSmithKline for damages on the grounds that they were not warned of the possible side-effects, such as personality changes, as well as addiction.
'The doctor told me it was not addictive and that's what it said on the information sheet,' says Gina, who lives near Morecambe in Lancashire.
Seroxat now carries the warning that some patients can have problems coming off it.
However, the Seroxat patients are a tiny proportion of the growing number of people addicted to prescription drugs.
It's thought that between three and seven million Britons are affected, with antidepressants, tranquillisers, sleeping pills and pain-killers the main culprits.
A recent report by the United Nations' International Narcotics Control Board predicted that the scale of the problem of addiction to legal drugs will soon overtake addiction to banned substances.
Yet despite this, few patients receive any effective help - meanwhile, millions of pounds are spent helping those addicted to illegal drugs. In fact, the problem of prescription addiction has, for years, been ignored or denied by drug companies and successive governments.
The move to sue GlaxoSmithKline is just one sign of growing pressure for some sort of action. Earlier this year, a House of Commons inquiry into addiction to prescription drugs concluded that action was needed.
FULL STORY
Read the new book, The Evidence, However, Is Clear...The Seroxat Scandal
By Bob Fiddaman
ISBN: 978-1-84991-120-7
CHIPMUNKA PUBLISHING
AVAILABLE FOR DOWNLOAD HERE
PAPERBACK COMING SOON
Monday, August 18, 2008
O/T AC/DC Fans Rock n' Roll Train
This guy from France (I think) does his best to show just how anticipated the new release is.
He deserves a Seroxat Sufferers Award - He's a legend and has brought a huge smile to my face.
Take a bow sir.
Fid
Read the new book, The Evidence, However, Is Clear...The Seroxat Scandal
By Bob Fiddaman
ISBN: 978-1-84991-120-7
CHIPMUNKA PUBLISHING
AVAILABLE FOR DOWNLOAD HERE
PAPERBACK COMING SOON
Body Count Linked to GSK Vaccine Trial Rises
Fourteen children have now died during a pneumonia vaccine trial run by GlaxoSmithKline in Argentina, according to the U.K.’s Daily Mail. Argentine authorities are investigating. GSK says the deaths are not linked to the tests.
This issue first emerged for GSK in July, when TradingMarkets.com reported that 12 babies had died during the trial. GSK has pointed out that 19,000 kids have received Synflorix so far and that the number of kids who have died is in fact lower than Argentina’s natural child mortality rate.
Read more here
Read the new book, The Evidence, However, Is Clear...The Seroxat Scandal
By Bob Fiddaman
ISBN: 978-1-84991-120-7
CHIPMUNKA PUBLISHING
AVAILABLE FOR DOWNLOAD HERE
PAPERBACK COMING SOON
Saturday, August 16, 2008
Seroxat - "The benefits far outweigh the risks"
1. It helps depressed people.
Here are some of the reported withdrawal effects [risks]
Paxil withdrawal Body
Paxil withdrawal Dry Mouth - The usual amount to moisture in the mouth is noticeably less.
Paxil withdrawal Sweating Increased - A large quantity of perspiration that is medically caused.
Paxil withdrawal Cardiovascular (Involving the heart and the blood vessels)
Read More in The Evidence, However, Is Clear...The Seroxat Scandal [The Ebook]
Chipmunka Publishing ISBN: 978-1-84991-120-7
Paperback coming soon
Friday, August 15, 2008
Don't Cry For Me GlaxoSmithKline
"Glaxo's trial includes thousands of babies in Argentina, where Alspach said 12 children died"
Argentina investigates deaths of vaccine kids
BUENOS AIRES, ARGENTINA (AP) - Argentine authorities are exploring a possible link between the deaths of 14 children and an experimental vaccine they were taking in a clinical trial run by GlaxoSmithKline.
Argentina's food and drug administration is investigating whether the deaths are tied to the Synflorix vaccine, said an agency official who spoke on condition of anonymity because he was not authorized to discuss the case.
The drug, designed to fight pneumonia, ear infections and several other pneumococcal diseases, was manufactured by the London-based GlaxoSmithKline PLC, the world's second-largest drug maker.
A U.S. spokeswoman for Glaxo, Sarah Alspach, said the company is not attributing the deaths to the experimental vaccine, which is being tested in three Latin American countries and in other countries around the world.
An independent board monitoring participants' safety recommended that the Latin American trials be temporarily suspended which they were in late June but then gave its OK for tests to resume, she added.
"We rely on their safety review," Alspach said. "Safety is our primary concern, always, with the development of any new treatment."
More than 19,000 babies have received at least one dose of Synflorix, which Glaxo plans to test on a total of 24,000 infants, she said. The company is still enrolling participants.
But according to the Argentine official, who works at the country's National Medicine, Food and Medical Technology Administration, the agency "received complaints about irregularities in the recruitment of patients" for the drug trial and on July 31 asked that recruitment be suspended.
Glaxo stopped recruiting the following day, saying it had already gathered the necessary number of participants, the official said.
Ana Maria Marchesse, who heads one of two groups that notified the national food and drug administration, told The Associated Press that she'd witnessed "poor ethical management" of patient recruitment.
"They didn't explain to the parents that this was an experimental vaccine, and a lot of the parents who signed consent forms were illiterate," said Marchesse, a pediatrician who heads the Health Professionals' Labor Association in the northern Argentine province of Santiago del Estero, where she said seven of the 14 children died.
"In some cases, they first gave them the vaccine and then gave them a 13-page consent form to sign that I had to read three times to understand," she added.
Marchesse said her group and a provincial doctors' association reported what they saw to the food and drug administration.
Glaxo's trial includes thousands of babies in Argentina, where Alspach said 12 children died; in Panama, where another two died; and in Chile. The natural infant death rate in those countries from pneumonia is 4 to 5 of every 1,000 live births, more than four times the rate seen in the study, Alspach said.
Pneumonia is the world's top killer among infectious diseases, causing more than 2 million deaths a year in children under five, mostly in developing countries, she said.
The company is testing the vaccine in more than 40 clinical studies around the world, she added. Data from other studies show the vaccine is about as safe and tolerable as competitor Wyeth's blockbuster Prevnar, a vaccine widely used against pneumococcal disease, she said.
Still, the Argentine province of Santiago del Estero is conducting a separate inquiry into the deaths of the seven children there, local Health Minister Franklin Moyano told state news media.
"While legal authorities investigate, we're in an observation phase to see if everything happened as expected, or if there were deviations that caused damage, in this case the death of seven kids," he said.
DEBORA REY ASSOCIATED PRESS
Originally published 11:36 p.m., August 14, 2008, updated 11:37 p.m., August 14, 2008
Read the new book, The Evidence, However, Is Clear...The Seroxat Scandal
By Bob Fiddaman
ISBN: 978-1-84991-120-7
CHIPMUNKA PUBLISHING
AVAILABLE FOR DOWNLOAD HERE
PAPERBACK COMING SOON
Thursday, August 14, 2008
Reasons For Change (Seroxat Ignorance)
Paxil Progress unites people from all corners of the world, they share stories, offer advice, post latest news stories related to Seroxat, the FDA, MHRA and GlaxoSmithKline. They vent their frustrations at the ignorance of health care workers, psychiatrists and GP's. [Doctors]
Many people are angry at Glaxo mouthpieces such as, Alistair Benbow and Mary Anne Rhyne who have publicly stated that withdrawal isn't an issue regarding Seroxat.
Glaxo and, it seems, the drug regulators, put the onus on doctors to help patients through withdrawal. A tactic of 'passing the buck' because they really don't have a clue what to do about this problem, they deny it exists or at least pass it off as part of the illness. Many doctors are faced with patients complaining of head zaps, violent thoughts, akathesia, suicidal thoughts et al.
A doctor has to make a decision that a manufacturer and regulator cannot make... or refuse to acknowledge. His decision will be based on his training. What doctor is trained in the art of SSRi withdrawal?
Many patients on Paxil Progress vent their anger at their doctors. They, like Glaxo and the regulators, just don't want to hear about withdrawal. It's easier to up the dosage, more cost effective too.
There are huge cracks that have, for years, been merely cemented over when it comes to Seroxat withdrawal. The glass building in Brent, London, stands hideously with the logo GlaxoSmithKline emblazoned at the top. There are cracks appearing in the glass at GSK HQ mainly because patients write about their ordeals at the hands of one of their best selling drugs. One such person to recently vent his frustrations was 'Dan' over at Paxil Progress. The title of Dan's post is 'Doctor's Appointment - Utterly Disgusted'.
Dan writes about his doctor and it echoes many stories I have heard throughout the years about the naivety and stubbornness but more importantly, lack of training in this field.
Dan complained that he was struggling with Seroxat withdrawal. His uneducated doctor suggests it's time to switch from Seroxat to another SSRi - herein lies the problem.
If the Seroxat Patient Information Leaflet continues to put the onus on uneducated doctors then this problem will never go away. More and more people will suffer because GlaxoSmithKline haven't got the balls or morals to do the right thing. The regulators are listening, they know how powerful blogging can be and they don't want their name further tarnished but listening is not good enough, they have to act.
The MHRA were literally bent over a barrel and buggered by GlaxoSmithKline regarding the suppression of data in the Seroxat paediatric studies. Later interviews with the then CEO of GSK, JP Garnier, would highlight just how smug these people can be. Alistair Benbow was in defiant mood as ever, claiming that the company had done nothing wrong - basically a middle finger salute to the four year MHRA Investigation.
I see this as a door opening for patients, a door that was once behind a brick wall that has been chipped away by bloggers.
GlaxoSmithKline's doors will always remain firmly closed to the public, their offer of putting clinical trial data on their web page is too little too late. They cannot be believed after hiding or manipulating data to market a dangerous drug in children. That was an act of cowardice, to defend it is an act utterly shameful.
It's time for change. Stories such as Dan's on Paxil Progress will help bring about that change.
Fid
Read the new book, The Evidence, However, Is Clear...The Seroxat Scandal
By Bob Fiddaman
ISBN: 978-1-84991-120-7
CHIPMUNKA PUBLISHING
AVAILABLE FOR DOWNLOAD HERE
PAPERBACK COMING SOON
Wednesday, August 13, 2008
Study of Paxil Use in Menopausal Women
Sponsors and Collaborators: Massachusetts General Hospital & GlaxoSmithKline
Information provided by: Massachusetts General Hospital
ClinicalTrials.gov Identifier: NCT00225914
Purpose
To evaluate the efficacy, safety, and tolerability of Paroxetine treatment in perimenopausal and postmenopausal women who present with menopause-related symptoms after discontinuing hormone therapy (HT), in the presence or absence of concomitant symptoms of depression or anxiety.
1: Experimental
Subjects enter into a six-week, double blind phase, randomized in a 1:1 ratio to paroxetine CR 12.5 mg/day; dosing may be adjusted up to 25 mg/day after two weeks, based on treatment response and tolerability.
Detailed Description:
This study is a 10-week double-blinded treatment study of perimenopausal and postmenopausal women who present with menopause-related symptoms after discontinuing Hormone Therapy(HT), with or without concomitant symptoms of depression and anxiety.
The menopausal transition is a period of heightened vulnerability to mood and anxiety disturbances. It is also a period when women may experience significant vasomotor symptoms (i.e. hot flushes and night sweats). More recently, the occurrence of vasomotor symptoms has been associated with increased risk for depression in menopausal women.
The efficacy of estrogens for the treatment of vasomotor symptoms is well established. In addition, the literature support a modulatory effect exerted by estrogen on various neurotransmitter systems that regulate mood and anxiety.
Despite the efficacy of hormone therapy (HT) for the treatment of menopause-related symptoms, a significant number of women discontinue its use during the first year of treatment. Moreover, recent findings from the Women's Health Initiative Study (WHI) have challenged the safety and the benefits that were initially thought to be associated with long-term use of HT. As a result, many women who have been taking HT decided to discontinue the use of HT, which may result in significant changes in their physical well being, quality of life and, possibly, their mental health status. Therefore, the efficacy and tolerability of other interventions such as antidepressants for these sub-populations warrant further investigation.
Treatment with Paroxetine has shown to be efficacious for menopause-related vasomotor symptoms. To date, no studies have examined the extent to which SSRIs may improve physical and psychological symptoms in women who discontinued HT.
Exclusion Criteria:
Women who present with moderate-to-severe symptoms of depression (MADRS scores > 19) or anxiety (BAI scores > 19) at baseline.
Women who meet diagnostic criteria at screening visit for a current major Axis I psychiatric disorder other than specific phobias (assessed through M.I.N.I. interview). Subjects presenting with symptoms of anxiety or depression, but not meeting criteria for Depressive Disorders, Bipolar Disorder, Panic Disorder, GAD, OCD or SAD, will be allowed in the study.
Regular treatment with hormonal medications, SSRIs, tricyclic antidepressant, mood stabilizer, oral neuroleptics, sedatives or hypnotics, over-the-counter agents known to influence hot flushes or mood within 4 weeks prior to screening visit; used of depot neuroleptics within 12 weeks prior to screening visit.
Suicidal ideation, homicidal ideation, or psychotic symptoms.
Menstrual dysfunction and amenorrhea of other etiologies.
History of seizure disorder
Pregnancy or breastfeeding.
Fid
Read the new book, The Evidence, However, Is Clear...The Seroxat Scandal
By Bob Fiddaman
ISBN: 978-1-84991-120-7
CHIPMUNKA PUBLISHING
AVAILABLE FOR DOWNLOAD HERE
PAPERBACK COMING SOON
Calling Korean Workers - Glaxo Want to 'Help' You
This is Phase IV of Glaxo's study.
Interestingly, Glaxo do not want any volunteers whom are a current homicidal or suicidal risk.
Makes you wonder why doesn't it?
Maybe Glaxo know paroxetine can push depressed people over the edge and don't want another lawsuit hanging over them?
I feel for the Koreans. They have a great work ethic and if something comes along to improve the chances of employees being able to work despite having a 'panic disorder' then they will embrace that opportunity.
I suspect there will be many Koreans wondering why they are shaking, sweating and having electric like sensations rip through their body in years to come. Meantime, Glaxo will yet again reap the rewards and deny that paroxetine has anything to do with the adverse reactions.
Mark my words Korea, you have some dark days ahead of you if you let GlaxoSmithKline push paroxetine onto your unsuspecting public.
Fid
Source:
Clinical Trials.gov Identifier: NCT00492414
Contacts
Contact: Eun-Joo Jung - menfipro@naver.com
Locations
Korea, Republic of
Seoul Paik Hospital,
Inje University
Sponsors and Collaborators
Inje University
GlaxoSmithKline Korea
Read the new book, The Evidence, However, Is Clear...The Seroxat Scandal
By Bob Fiddaman
ISBN: 978-1-84991-120-7
CHIPMUNKA PUBLISHING
AVAILABLE FOR DOWNLOAD HERE
PAPERBACK COMING SOON
Tuesday, August 12, 2008
Paroxetine and Deformed Children

Thanks to the anonymous 'tipster' for sending me recent files.
The basic message in this one was this:
The present findings are consistent with other recent results suggesting the possibility of a modestly increased occurrence of congenital malformations following first trimester exposure to paroxetine compared to ,other antidepressants.
Paroxetine in the first trimester and the prevalence of congenital malformations
J. Alexander Cole DSc, MPH1*,y, Sara A. Ephross PhD2,
Irene S. Cosmatos MS3 and Alexander M. Walker MD, DrPH1
1 i3 Drug Safety, Auburndale, MA, USA
2 GlaxoSmithKline, Research Triangle Park, NC, USA
3 GlaxoSmithKline, Philadelphia, PA, USA
SUMMARY
Purpose To refine a preliminary analysis identifying a possibly increased prevalence of malformations among infants born to women exposed to paroxetine in the first trimester.
Methods This study used data from UnitedHealthcare, a large U.S. insurer, using datasets originally for a study of bupropion in pregnancy. We identified women with a live-born delivery between January 1995 and September 2004. We classified women according to their first trimester mono- or mono/polytherapy exposure to paroxetine and other antidepressants. We confirmed malformation cases by medical record abstraction. We calculated the adjusted odds ratios (AORs) through logistic regression.
Conclusions These more detailed paroxetine findings confirm previous findings of analyses of these data among women exposed to all types of antidepressants. The present findings are consistent with other recent results suggesting the possibility of a modestly increased occurrence of congenital malformations following first trimester exposure to paroxetine compared to other antidepressants. Copyright # 2007 John Wiley & Sons, Ltd.
Fid
Read the new book, The Evidence, However, Is Clear...The Seroxat Scandal
By Bob Fiddaman
ISBN: 978-1-84991-120-7
CHIPMUNKA PUBLISHING
AVAILABLE FOR DOWNLOAD HERE
PAPERBACK COMING SOON
Monday, August 11, 2008
Young Girls Abused In Care!
The blurb reads thus:
Thrust into care at six months of age because of an alcoholic father and mentally ill mother, Teresa Cooper's life began in a less than perfect way. Teresa spent an unsettled childhood in a variety of children's homes before being sent to Kendall House in Kent, which would become her prison and worst nightmare. At Kendall House, Teresa became a victim of a terrible regime, being injected with dangerously high doses of drugs and sexually abused. This cruel and vicious treatment, accompanied by punishments such as 163 days spent in solitary confinement, meant that it was not long before Teresa began to harm herself and even attempt to take her life. After three years of hell, Teresa thought her nightmare was over but another was about to begin. Teresa Cooper is a survivor. Fighting against a corrupt social care system, she has taken her case of abuse and drugging to parliament, and is fighting to prevent many more children from suffering at the hands of unethical doctors and abusive foster parents.
Teresa has now petitioned the UK Government to look in to this matter - I've just signed - I urge you all to follow suit. HERE
Fid
Read the new book, The Evidence, However, Is Clear...The Seroxat Scandal
By Bob Fiddaman
ISBN: 978-1-84991-120-7
CHIPMUNKA PUBLISHING
AVAILABLE FOR DOWNLOAD HERE
PAPERBACK COMING SOON
Sydney Psychiatrist 'Poo Poo's' Patient Experience
Her book 'Dying For A Cure' has been on sale in Australia for over a year now and will shortly be launched here in the UK.
Yesterday I recieved an email from an Australian who had stumbled upon my blog. They pointed me in the direction of a critique left by Michael Robertson, a psychiatrist from Sydney, Australia.
Robertson goes about disecting Rebekah Beddoe's experience with what we [SSRi sufferers] have come to expect from those who would rather blame the illness than the drug.
His opening para sets a precedent for his rebuke of Beddoe's experience:
Dying for a Cure reads like a prolonged formal complaint, rather than a considered piece on this controversial area. Rebekah Beddoe makes no attempt to conceal her contempt for the psychiatrists who tried to care for her during her crisis. The book depicts psychiatrists as either lecherous and hapless, or arrogant and officious. Regardless of the accuracy of these portraits, her frank account of her life story betrays a deeper problem which it would seem explains some of her anger. In describing the challenges motherhood presented her psyche, she invites us into the poisonous transference responses which lurk throughout the book’s narrative:
Robertson, also appears to have a dig at Breggin and Healy. He writes:
The book is quite well researched all the main scientific papers addressing the adverse effects of newer antidepressants are mentioned, although this scholarly dimension is undermined by the book’s overemphasis on the opinions of SSRI critics like Peter Breggin and David Healy. Indeed, this incessant use of the rhetorical device of ‘appeals to authority’ weakens Beddoe’s arguments considerably. Beddoe proffers little more than straw-man arguments against psychiatry delivered in an excessively contemptuous tone.
It was hardly surprising to learn that Robertson has recieved over $2 Million in grants from, as yet, unknown sources.
Maybe this rather large sum of money he has recieved 'weakens his argument considerably'
I wrote to Robertson but as yet I have not had a response.
I will keep you posted if he responds.
Fid
Read the new book, The Evidence, However, Is Clear...The Seroxat Scandal
By Bob Fiddaman
ISBN: 978-1-84991-120-7
CHIPMUNKA PUBLISHING
AVAILABLE FOR DOWNLOAD HERE
PAPERBACK COMING SOON
MEETING WITH THE MHRA

I choose to do this because of a rather insidious character who helps moderate, it seems, a mental health forum. It seems this person is either infatuated with me or has nothing better to do with his life.
A recent visit to his forum sees a discussion labelling me a 'Scientologist lover'
More recently he appeared on another website under the guise of someone else and tried to discredit many more Seroxat campaigners. He also taunted me by saying that I hadn't got a meeting with the MHRA!
Well, I have that meeting now 'Jeremy' - I may invite my 'Scientologist friends' seeing as I love them so much.
The meeting will take place sometime in September. For the record, I will be paying my own travelling expenses.
Fid
Read the new book, The Evidence, However, Is Clear...The Seroxat Scandal
By Bob Fiddaman
ISBN: 978-1-84991-120-7
CHIPMUNKA PUBLISHING
AVAILABLE FOR DOWNLOAD HERE
PAPERBACK COMING SOON
Sunday, August 10, 2008
Seroxat : The Situation As It Stands : August 2008
Truthman hasn't written anything on his blog for almost a year but believe me he works tirelessly in the background. Never a day goes by where he hasn't sent me links to various articles in the press or hidden away somewhere on the Internet.
Truthman rounds up the past year regarding Seroxat, the MHRA and GlaxoSmithKline in a piece worthy of publication in one of Britain's newspapers.
Highly recommended reading.
Personally, I have some good news that I will share with you all tomorrow regarding my insistence on meeting with Kent Woods, CEO of the MHRA. Meantime, check out the Truthman's summary of the last 12 months HERE
Fid
Read the new book, The Evidence, However, Is Clear...The Seroxat Scandal
By Bob Fiddaman
ISBN: 978-1-84991-120-7
CHIPMUNKA PUBLISHING
AVAILABLE FOR DOWNLOAD HERE
PAPERBACK COMING SOON
COMING SOON - NEW PAXIL/SEROXAT/AROPAX WEBSITE
Fid
Read the new book, The Evidence, However, Is Clear...The Seroxat Scandal
By Bob Fiddaman
ISBN: 978-1-84991-120-7
CHIPMUNKA PUBLISHING
AVAILABLE FOR DOWNLOAD HERE
PAPERBACK COMING SOON
Saturday, August 09, 2008
COMING SOON - NEW WEBSITE
To be hosted on an ever increasing popular Networking site.
GSK WOULDN'T LISTEN
SO OTHERS HAVE TO BE TOLD
"We just stepped up a gear, it's time for GSK to suffer like those who have suffered at the hands of Seroxat/Paxil/Aropax"
Read the new book, The Evidence, However, Is Clear...The Seroxat Scandal
By Bob Fiddaman
ISBN: 978-1-84991-120-7
CHIPMUNKA PUBLISHING
AVAILABLE FOR DOWNLOAD HERE
PAPERBACK COMING SOON
DYING FOR A CURE
Shortly after the birth of her daughter Rebekah Beddoe was diagnosed with post-natal depression. Two years later she was taking six different drugs, including lithium, a tranquilliser, an antipsychotic, and antidepressants. She had been diagnosed with bipolar disorder; given electric-shock therapy; made numerous attempts on her life; and was alternately manic and consumed by crippling despair during which she could barely move. She had a two-year-old daughter she hardly knew and a mother and partner who were at their wits' end, unable to recognise the formerly ambitious, vibrant and highly successful woman they loved so much.
Australians have embraced antidepressants: twelve million prescriptions are written annually, mostly by GPs. But, what do we really know of the pills' effects? The idea that they correct a chemical imbalance in our brain is by no means proven, indeed there is much evidence that contradicts this view. It is commonly thought such drugs are not addictive; in fact, as Rebekah found to her great distress, they are hard to come off and those who do may suffer debilitating side effects.
This is a powerful memoir of the nightmarish three years Rebekah endured as she was repeatedly misdiagnosed, only to realise that her medication was the cause of her mental deterioration. In this book Rebekah calls for better information from the pharmaceutical companies about the risks associated with antidepressants and similar classes of drugs - facts, rather than marketing dressed up as medical science - and for a re-examination of the ways some psychiatrists treat their patients.
Australians can purchase the book HERE
Read the new book, The Evidence, However, Is Clear...The Seroxat Scandal
By Bob Fiddaman
ISBN: 978-1-84991-120-7
CHIPMUNKA PUBLISHING
AVAILABLE FOR DOWNLOAD HERE
PAPERBACK COMING SOON
Friday, August 08, 2008
GSK Duped Norwegian Medicinal Agency it seems.
 Not content with duping the FDA and the MHRA, evidence has now come to light that GlaxoSmithKline had not reported to and had misrepresented the frequency of adverse reactions that were noted in the early clinical trials [Paroxetine] – to the Norwegian Medicinal Agency! {1}
Not content with duping the FDA and the MHRA, evidence has now come to light that GlaxoSmithKline had not reported to and had misrepresented the frequency of adverse reactions that were noted in the early clinical trials [Paroxetine] – to the Norwegian Medicinal Agency! {1}Adverse drug reactions are of particular interest when they have been clandestine for many years. We were admitted access to unpublished data through the Civil Ombudsman’s unusual decision to advise the Norwegian Ministry of Health to let us examine files in the archives of the Norwegian Medicinal Agency. We found that reports of a series of randomized controlled studies evaluating the efficacy and safety of paroxetine already existed at the time of the first application for marketing authorization. Common to these studies were that they were small, were of short duration and were mostly published individually. Some of them have remained unpublished and no thorough meta-analysis has been carried out either for efficacy data or for adverse event data. The main published conclusions from these studies are that paroxetine is a safe and effective medication for the treatment of major depression.2–7
During the widely clinical use of paroxetine, more adverse drug reactions have become evident than were evident when the drug was first licensed in 1989. Our hypothesis was that some of these adverse drug reactions were already documented in the initial clinical trials forming part of the application for marketing authorization, but that no adequate pooling or meta-analysis of the data was carried out. We therefore wanted to explore the usefulness of gathering information about the occurrence of adverse reactions to a drug by means of randomized controlled trials at the time of first application for marketing authorization and to analyse these data by a meta analysis.
We wanted to determine to what extent adverse drug effects associated with a selective serotonin reuptake inhibitor (SSRI) were known but not assessed before application for registration of paroxetine.
Methods With a special permit from the Norwegian Ministry of Health, we obtained reports from 82 clinical trials presented by the paroxetine license holder in 1989. There were 17 double blind, placebo controlled clinical trials with parallel design with 903 patients on paroxetine and 592 on placebo. Altogether 32 adverse effects showed a risk difference (RD) between paroxetine and control groups of more than 0.5%.We did a meta-analysis for each of these adverse effects. We then compared the outcome with the frequencies stated in the Summary of Product Characteristics (SPC) at the time of registration and those reported in the current SPC.
At the time of registration 19 of the adverse effects were statistically significant. Only eight of these adverse effects were listed as being common in the first SPC from 1989. Five out of the nineteen adverse effects are not mentioned in the current SPC. Among them are headache with RD 5.4%, decreased libido RD 2.6%, nervousness RD 2.0% and paresthesia (brain zaps) RD 1.7%.
Frequently occurring adverse reactions that are included in today’s SPC for paroxetine were evident and documented already in the early studies accompanying the application for marketing authorization in 1989. Some other adverse effects observed then are still not mentioned in the SPC of today. Meta-analyses of adverse effects should be mandatory at the stage of first registration of a drug.
I see from my webstats that GlaxoSmithKline from Hertford, UK, have been looking in today.
I have a message for you:
You manufactured and marketed a drug that I took. Because you never reported these adverse events at the time I took it, I put my faith in you, I trusted you. I have since learned that you not only duped me but our regulator too, and now it seems, the Norwegian regulator!
It's my mission to make sure you don't dupe others, be they regulators, doctors or more importantly, patients.
Do you want me to go away Glaxo?
See me as your confessional box, although I won't be telling you to go away and say three Hail Mary's for your sins.
I'm here to stay.
GET USED TO IT.
Fid
Read the new book, The Evidence, However, Is Clear...The Seroxat Scandal
By Bob Fiddaman
ISBN: 978-1-84991-120-7
CHIPMUNKA PUBLISHING
AVAILABLE FOR DOWNLOAD HERE
PAPERBACK COMING SOON
Thursday, August 07, 2008
Paroxetine effects on emotions
It's nice to see a group of healthy psychologists and psychiatrists using themselves rather than 'subjects' in this study.
Basically, the outcome was thus:
In the paroxetine groups, a total of thirty two psychiatric adverse events were recorded, with the authors concluding that they possibly were related to the paroxetine treatment.
There were four psychiatric adverse events recorded in the placebo (sugar pill) group!
Now... DO THE BENEFITS OUTWEIGH THE RISKS?
Paroxetine effects on emotions: a 4-week randomized placebo-controlled pilot study in psychologists and psychiatrists
Purpose of the study: The impact of paroxetine on emotional functioning is questioned, with regards to the known implication of serotonine in emotional regulation. The objective of our study was to evaluate the effects of paroxetine on emotional functioning in three arms: double blind paroxetine (DBPX), single-blind paroxetine (SBPX) and double-blind placebo (DBPL). Healthy psychologists and psychiatrists were elected for their ability to analyze with a correct sensibility changes in their emotions.
Method: 30 healthy participants (mean age: 33.5, gender ratio F/M: 1.5) working as psychiatrists (N = 18) or psychologists (N = 12) were randomly assigned to receive an ambulatory treatment with paroxetine (DBPX: N= 10, SBPX: N= 10) or placebo (DBPL: N= 10). Paroxetine was administered for 4 weeks at 20 mg/day. Emotional functioning was evaluated on D0, D7, D14 and D28 with the Emotional State Questionnaire (ESQ), a self questionnaire designed to assess 4 emotional dimensions: “recognition”, “expression”, “internal emotional experience” and “social context” [1]. The primary assessment criteria were ESQ scores on D28 compared with those on baseline. Impact of paroxetine on anxiety and depression was assessed by the Spielberger State-Trait Anxiety Inventory (STAI) and the Center for Epidemiologic Studies Depression Scale (CES-D). The Addiction Research Center Inventory (ARCI [2]), based on drug users’ experience, was performed to measure euphoria, dysphoria, sedation and stimulant effects. Changes on D28 from baseline were compared between treatment groups, gender and professional status.
Conclusions: This is the first study to assess the impact of a 4-week paroxetine treatment on emotional functioning in a healthy psychiatrists and psychologists population. DBPX was distinguishable from the other groups by a decrease in the internal emotional experience of ESQ. Concordant with this result, DBPX reported more sedation and less stimulant effects. Moreover, differences between DBPX and SBPX were observed in both psychological evaluation and tolerance. Two factors could be involved in the clinical response to paroxetine in patients: a decrease in emotional feeling and treatment awareness.
References
[1] Catherine Cass´e-Perrot Eric Fakra Elisabeth Jouve Olivier Blin Conceptualisation and Validation of the “Emotional State Questionnaire (ESQ)”: Evaluation of an Emotional Profile L’Enc´ephale (in press)
[2] Warot D, Danjou P, Payan C, Puech A-J, 1997, Sensitivity and specificity to amphetamine of a french version of the 49-item form of the addiction research center inventory. Drug and Alcohol Dependence 45, 177–183.
Fid
Read the new book, The Evidence, However, Is Clear...The Seroxat Scandal
By Bob Fiddaman
ISBN: 978-1-84991-120-7
CHIPMUNKA PUBLISHING
AVAILABLE FOR DOWNLOAD HERE
PAPERBACK COMING SOON




